What are Amines?
Amines are a fundamental class of organic compounds that play a crucial role in various aspects of chemistry and biology. They are essentially derivatives of ammonia (NH3), where one or more hydrogen atoms are replaced by alkyl or aryl groups. This substitution gives rise to a fascinating array of properties and applications for amines.
Imagine ammonia, a simple molecule with three hydrogen atoms attached to a nitrogen atom. Now, picture replacing one of those hydrogen atoms with a carbon-containing group – that’s the basic idea behind amines. We can even swap out more than one hydrogen atom, leading to different types of amines.
Think of amines like versatile building blocks in the world of organic chemistry. They can be found in natural products, pharmaceuticals, and even everyday materials.
Classifying Amines: A Tale of Primary, Secondary, and Tertiary
Amines are meticulously categorized based on the number of hydrogen atoms in ammonia that have been replaced by alkyl or aryl groups. This classification helps us understand their reactivity and properties:
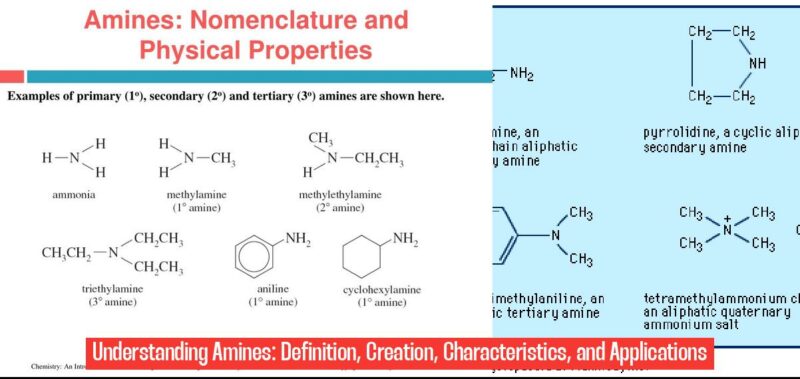
- Primary Amines (RNH2): These amines have one hydrogen atom replaced by an alkyl or aryl group. Think of them as the simplest form, with a single “branch” extending from the nitrogen atom.
- Secondary Amines (R2NH): With two hydrogen atoms replaced, secondary amines have two “branches” coming off the nitrogen.
- Tertiary Amines (R3N): These amines have all three hydrogen atoms replaced, resulting in a nitrogen atom surrounded by three “branches.”
To illustrate this, consider the following examples:
- Methylamine (CH3NH2): A primary amine with a single methyl group (CH3) attached to the nitrogen.
- Dimethylamine ((CH3)2NH): A secondary amine with two methyl groups attached to the nitrogen.
- Trimethylamine ((CH3)3N): A tertiary amine with three methyl groups attached to the nitrogen.
These classifications are not just theoretical concepts; they have real-world implications. For instance, primary amines are often used as starting materials for the synthesis of other important compounds, while tertiary amines find applications in pharmaceuticals and dyes.
Nomenclature of Amines: A System for Naming
Just like any other chemical compound, amines need a systematic way of naming them. The International Union of Pure and Applied Chemistry (IUPAC) provides a clear and unambiguous system for naming amines.
In the IUPAC system, amines are generally named as alkanamines. This involves taking the name of the parent alkane and adding the suffix “amine,” replacing the “e” at the end of the alkane name. For example, CH3NH2 is named methanamine, derived from the alkane methane.
However, when naming arylamines, the suffix “e” of the arene is replaced with “amine.” For instance, C6H5NH2 is named benzenamine, while C6H5CH2NH2 is known as phenylmethanamine.
Beyond IUPAC: Common Names and Functional Groups
While IUPAC nomenclature provides a standardized way of naming amines, there are also common names and functional group names that are widely used. For example, CH3NH2 is often called methylamine, and C6H5NH2 is frequently referred to as aniline.
Functional groups are specific arrangements of atoms within a molecule that often determine its chemical behavior. In the case of amines, the amino group (-NH2) is the key functional group that defines their properties.
Understanding the naming conventions for amines is essential for comprehending their structure, properties, and relationships to other organic compounds.
Preparation of Amines: Creating These Versatile Compounds
Now that we’ve explored the basics of amines, let’s delve into how these compounds are made. The synthesis of amines involves a range of chemical reactions, each with its own advantages and limitations.
Here are some common methods for preparing amines:
1. Reduction of Nitro Compounds: A Transformation from Nitro to Amino
Nitro compounds (R-NO2) are organic compounds containing a nitro group (-NO2). This method is a classic way to convert a nitro group into an amino group (-NH2). The process involves exposing the nitro compound to hydrogen gas (H2) in the presence of a catalyst, typically a metal like nickel, palladium, or platinum.
For instance, nitrobenzene (C6H5NO2) can be reduced to aniline (C6H5NH2) using this technique:
C<sub>6</sub>H<sub>5</sub>NO<sub>2</sub> + 3H<sub>2</sub> → C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub> + 2H<sub>2</sub>O
This reaction is a fundamental step in the synthesis of many important amines, including pharmaceuticals and dyes.
2. Ammonolysis of Alkyl Halides: A Nucleophilic Substitution
Ammonolysis involves the reaction of an alkyl halide (R-X, where X is a halogen) with ammonia (NH3). This reaction is a nucleophilic substitution, where the ammonia molecule attacks the carbon atom bonded to the halogen, displacing the halogen and forming an amine.
A simple example is the reaction of methyl chloride (CH3Cl) with ammonia to form methylamine (CH3NH2):
CH<sub>3</sub>Cl + NH<sub>3</sub> → CH<sub>3</sub>NH<sub>2</sub> + HCl
This reaction is often used to produce various primary amines, but it can also lead to the formation of secondary and tertiary amines as byproducts.
3. Reduction of Nitriles: Adding a Carbon
Nitriles (R-CN) are organic compounds containing a cyano group (-CN). The reduction of nitriles provides a convenient method for preparing primary amines with one additional carbon atom compared to the starting nitrile. This reduction can be carried out using lithium aluminium hydride (LiAlH4) or catalytic hydrogenation.
For example, the reduction of acetonitrile (CH3CN) using LiAlH4 gives ethylamine (CH3CH2NH2):
CH<sub>3</sub>CN + 4[H] → CH<sub>3</sub>CH<sub>2</sub>NH<sub>2</sub>
This reaction is useful for synthesizing amines with specific carbon chain lengths.
4. Reduction of Amides: Converting Amides to Amines
Amides (R-CONH2) are organic compounds containing a carbonyl group (C=O) bonded to a nitrogen atom. The reduction of amides, using a strong reducing agent like lithium aluminium hydride (LiAlH4), yields amines.
For instance, the reduction of acetamide (CH3CONH2) with LiAlH4 produces ethylamine (CH3CH2NH2):
CH<sub>3</sub>CONH<sub>2</sub> + 4[H] → CH<sub>3</sub>CH<sub>2</sub>NH<sub>2</sub> + H<sub>2</sub>O
This method is particularly valuable for preparing amines from readily available amides.
5. Gabriel Phthalimide Synthesis: A Route to Primary Amines
The Gabriel phthalimide synthesis is a specific and elegant method for preparing primary amines. This method utilizes phthalimide, a cyclic compound containing an imide group, as a key reagent.
The process involves:
- Formation of Potassium Phthalimide: Phthalimide is treated with an ethanolic solution of potassium hydroxide (KOH) to form potassium phthalimide.
- Alkylation: The potassium phthalimide is then reacted with an alkyl halide (R-X) to form an N-alkyl phthalimide.
- Hydrolysis: The N-alkyl phthalimide is hydrolyzed with a strong base, such as KOH, to release the primary amine.
This reaction sequence is particularly useful for preparing primary amines where other methods might lead to unwanted byproducts.
Physical Properties of Amines: From Gases to Liquids
Amines exhibit a range of physical properties, influenced by factors like their structure, molecular weight, and the ability to form hydrogen bonds.
- Solubility: Lower aliphatic amines, those with short carbon chains, are generally soluble in water due to their ability to form hydrogen bonds with water molecules. However, as the carbon chain length increases, the solubility decreases. Tertiary amines, with no hydrogen atoms directly attached to the nitrogen, show reduced solubility due to the absence of hydrogen bonding.
- Boiling Point: Like solubility, boiling points of amines are also affected by hydrogen bonding. Primary and secondary amines have higher boiling points than tertiary amines due to the presence of hydrogen bonding between molecules. The stronger the hydrogen bonding, the more energy it takes to break the intermolecular forces, resulting in a higher boiling point.
- Odor: Lower aliphatic amines often have a characteristic fishy odor, while aromatic amines generally have a distinctive, pungent smell.
Amines and Their Impact on Our Senses
The odor of amines is often described as “fishy” because they are produced during the decomposition of fish and other organic matter. This odor is actually due to the breakdown of amino acids, which contain amine groups.
The fishy odor of amines can be a good indication of spoilage in food. If you notice a strong fishy smell from fish, it’s a sign that it’s no longer fresh.
Hydrogen Bonding: A Key to Understanding Properties
Hydrogen bonding is a special type of intermolecular force that occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This strong interaction is responsible for the unique properties of water, and it also plays a significant role in the behavior of amines.
In primary and secondary amines, the hydrogen atom attached to the nitrogen can form hydrogen bonds with other amine molecules. This hydrogen bonding contributes to their higher boiling points and solubility in water.
Tertiary amines, on the other hand, lack the hydrogen atom directly attached to the nitrogen, limiting their ability to form hydrogen bonds. This explains their lower boiling points and reduced solubility in water.
Chemical Properties of Amines: The Reactivity of Nitrogen
Amines are known for their reactivity, particularly their basic character. This arises from the presence of the lone pair of electrons on the nitrogen atom.
1. Basicity: The Ability to Accept a Proton
Amines act as Brønsted-Lowry bases, meaning they can accept a proton (H+) from an acid, forming an ammonium salt. The basicity of amines is influenced by factors like the electron-donating or withdrawing nature of the groups attached to the nitrogen atom.
For instance, aliphatic amines are generally more basic than aromatic amines. This difference arises because alkyl groups (R) are electron-donating, increasing the electron density on the nitrogen atom and making it more likely to accept a proton.
2. Alkylation: Adding Alkyl Groups to the Nitrogen
Alkylation is a reaction where an alkyl group (R) is added to the nitrogen atom of an amine. This reaction is often used to synthesize secondary and tertiary amines from primary amines.
For example, the reaction of methylamine (CH3NH2) with methyl iodide (CH3I) forms dimethylamine ((CH3)2NH):
CH<sub>3</sub>NH<sub>2</sub> + CH<sub>3</sub>I → (CH<sub>3</sub>)<sub>2</sub>NH + HI
Alkylation reactions are important in organic synthesis, allowing chemists to modify the structure and properties of amines.
3. Acylation: Introducing an Acyl Group
Acylation is a reaction in which an acyl group (R-C=O) is added to the nitrogen atom of an amine. This reaction is commonly used to synthesize amides, which are important compounds in pharmaceuticals, polymers, and other industries.
For example, the reaction of methylamine (CH3NH2) with acetyl chloride (CH3COCl) forms N-methylacetamide (CH3CONHCH3):
CH<sub>3</sub>NH<sub>2</sub> + CH<sub>3</sub>COCl → CH<sub>3</sub>CONHCH<sub>3</sub> + HCl
Acylation reactions are fundamental in organic synthesis, enabling the formation of new carbon-nitrogen bonds and creating diverse amide structures.
4. Carbylamine Reaction: Distinguishing Primary Amines
The carbylamine reaction, also known as the isocyanide test, is a unique test for identifying primary amines. In this reaction, a primary amine is heated with chloroform (CHCl3) in the presence of a strong base, such as potassium hydroxide (KOH), to produce a foul-smelling isocyanide (R-NC).
For example, the reaction of methylamine (CH3NH2) with chloroform and KOH produces methyl isocyanide (CH3NC):
CH<sub>3</sub>NH<sub>2</sub> + CHCl<sub>3</sub> + 3KOH → CH<sub>3</sub>NC + 3KCl + 3H<sub>2</sub>O
This reaction is highly specific for primary amines, making it a valuable tool for qualitative analysis.
Uses of Amines: A Wide Range of Applications
Amines are incredibly versatile compounds with a wide range of applications across various industries, including pharmaceuticals, agriculture, and manufacturing.
1. Pharmaceuticals: Amines in Medicine
Amines play a vital role in the pharmaceutical industry, where they are used as starting materials for synthesizing various drugs. Many drugs contain amine functional groups, which contribute to their pharmacological activity.
- Antidepressants: Some antidepressants, such as sertraline and fluoxetine, contain amine functional groups that interact with neurotransmitters in the brain to regulate mood.
- Pain Relievers: Analgesics like morphine and demerol, which are used to relieve pain, also contain amine groups.
- Antihistamines: Antihistamines, like diphenhydramine (found in Benadryl), contain tertiary amine groups that block the action of histamine, a chemical involved in allergic reactions.
2. Agriculture: Amines in Crop Production
Amines are used in agriculture as herbicides, pesticides, and fungicides. These compounds help control weeds, pests, and diseases, improving crop yields and protecting plants from damage.
- Herbicides: Some herbicides, like glyphosate, contain amine groups that disrupt the growth of weeds.
- Pesticides: Insecticides like imidacloprid, which is used to control insect pests, also contain amine functional groups.
3. Manufacturing: Amines in Various Industries
Amines find numerous applications in manufacturing processes.
- Dyes: Amines are essential starting materials in the production of dyes. Many dyes contain amine groups, which contribute to their color and binding properties.
- Polymers: Amines are used in the synthesis of polymers, which are large molecules composed of repeating subunits. For example, polyamides, such as nylon, are produced by reacting diamines with diacids.
- Rubber: Amines are used in the vulcanization of rubber, a process that cross-links rubber molecules to improve their strength and elasticity.
4. Other Uses: Beyond the Obvious
Amines also have various other uses:
- Food Additives: Some amines are used as food additives, such as flavoring agents and preservatives.
- Cosmetics: Amines are found in cosmetics, such as hair dyes and shampoos.
- Explosives: Some amines are used in the production of explosives, such as dynamite.
Conclusion: Amines, A Fundamental Class of Compounds
Amines are a fundamental class of organic compounds with diverse properties and applications. Their unique structure, reactivity, and ability to form hydrogen bonds make them essential components in various fields, from pharmaceuticals to agriculture and manufacturing.
By understanding the principles of amine chemistry, we gain insights into their role in the world around us, from the medicines we take to the food we eat.
This exploration of amines has revealed the remarkable versatility and importance of this class of compounds, highlighting their significance in both the natural and synthetic world. From the basic principles of their structure and naming to their intricate chemical properties and wide-ranging applications, amines continue to fascinate and inspire chemists and scientists alike.
What are amines?
Amines are a fundamental class of organic compounds derived from ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups.
How are amines classified?
Amines are classified as primary, secondary, or tertiary based on the number of hydrogen atoms in ammonia that have been replaced by alkyl or aryl groups.
Can you provide examples of primary, secondary, and tertiary amines?
Examples include Methylamine (CH3NH2) as a primary amine, Dimethylamine ((CH3)2NH) as a secondary amine, and Trimethylamine ((CH3)3N) as a tertiary amine.
What are some applications of primary and tertiary amines?
Primary amines are often used as starting materials for the synthesis of other important compounds, while tertiary amines find applications in pharmaceuticals.